Increasing lawsuits by women over Paragard Copper IUD complications during removal have drawn attention to this widely used contraceptive device. These lawsuits allege that the manufacturers failed to adequately warn users about the risks associated with its removal.
According to Forbes, most lawsuits are product liability claims based on an alleged design defect of the Paragard IUD. These lawsuits allege negligence on the part of the manufacturer. Those eligible for the lawsuit are women whose Paragard IUD broke either before or during removal, leading to complications.
In this article, we delve into the complexities of these Paragard IUD lawsuits and the legal challenges they present.
The Rise in Paragard Lawsuits
According to TorHoerman Law, there has been a significant increase in Paragard lawsuits in recent years. Thousands of women are pursuing legal action currently. According to an August 2024 update by ConsumerNotice.org, there are 2,094 lawsuits pending in the Paragard IUD MDL.
The primary reason for this surge is the reported complications experienced during the removal of the Paragard IUD. Women have faced issues such as breakage of the device, pieces becoming lodged in their bodies, and severe pain.
As the number of Paragard lawsuits continues to rise, it highlights the importance of addressing potential safety concerns. The complexity of these cases has made them a focal point in product liability litigation.
Allegations of Negligence
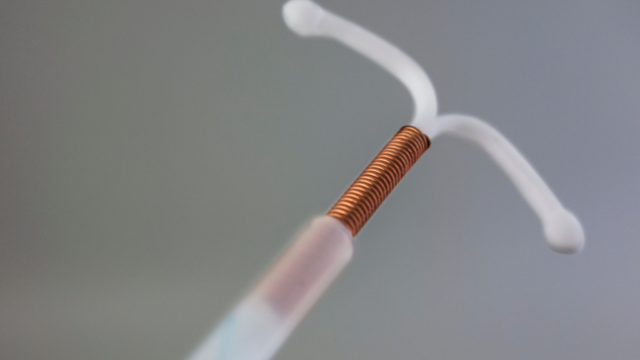
One of the central claims in these lawsuits is that CooperSurgical and Teva Pharmaceuticals were negligent in marketing Paragard. Plaintiffs argue that the manufacturers failed in their duty to provide adequate warnings about the potential risks and complications.
The allegations of negligence raise questions about the responsibility of medical device manufacturers to ensure the safety of their products. The outcome of these lawsuits may have far-reaching implications for similar cases involving medical devices.
Manufacturing Defect Claims
Another critical aspect of these lawsuits revolves around the assertion that Paragard IUDs have manufacturing defects. Plaintiffs argue that these defects have contributed to the device’s tendency to break during removal, leading to serious injuries.
The claims of manufacturing defects highlight the need for rigorous quality control in the production of medical devices. Manufacturers must meet high standards to ensure the safety of patients who rely on their products.
Defective Design Concerns
The debate over whether the design of the Paragard IUD is fundamentally flawed forms a crucial element of these lawsuits. Plaintiffs contend that the device’s design makes it more prone to breakage during removal, causing harm to users.
The discussion surrounding the device’s design underscores the importance of thorough engineering and testing in the development of medical devices. It also casts doubt on the device’s initial evaluations.
Insufficient Warning Labels
Insufficient warning labels have been a key point of contention in the Paragard IUD lawsuit. Plaintiffs argue that the warnings provided with the device were not clear enough to inform users of the potential risks.
This lack of adequate warning labels is seen as a significant factor contributing to the injuries experienced by many women. This has prompted a deeper look at the medical device industry’s labeling policies.
As reported by CBS12, Paragard did modify the label in 2019 to include updated details regarding breakage. Nevertheless, Fort Lauderdale lawyer Marcus Susen observed that this action came too slowly. Numerous women had already experienced adverse effects by that time.
FDA Involvement
The FDA’s role in monitoring the safety of medical devices like Paragard has been central to these lawsuits. Plaintiffs have highlighted instances where the FDA issued warnings or took action related to the device’s safety.
The FDA’s involvement in this litigation highlights the importance of regulatory oversight in the healthcare industry. It also emphasizes the importance of constant monitoring of medical equipment.
What Lies Ahead
As the Paragard IUD lawsuits progress, there is uncertainty regarding the outcomes. Legal experts anticipate a lengthy legal process as both sides present their arguments.
The next step might be a combination of negotiations and trials. There is potential for significant financial implications for manufacturers and potential compensation for affected women.
The outcome is highly anticipated, as it will potentially establish legal standards for future medical device product liability cases.

Final Word
The surge in Paragard IUD lawsuits underscores the significance of addressing safety concerns within the medical device industry. These complex cases highlight issues of negligence, manufacturing defects, and design flaws. This emphasizes the need for rigorous quality control and clear warning labels.
The FDA’s role in this litigation highlights the importance of regulatory oversight. As these lawsuits evolve they could set guidelines for similar claims involving medical devices in the future. This impacts both manufacturers and the safety of patients. The uncertainty surrounding the lawsuit outcomes highlights the ongoing need for vigilance in ensuring the safety of medical devices.